*Base substitutions, insertions or deletions, copy number alterations and gene rearrangements.
†309 genes with complete coding exonic coverage, 15 genes with select intronic or non-coding regions only.
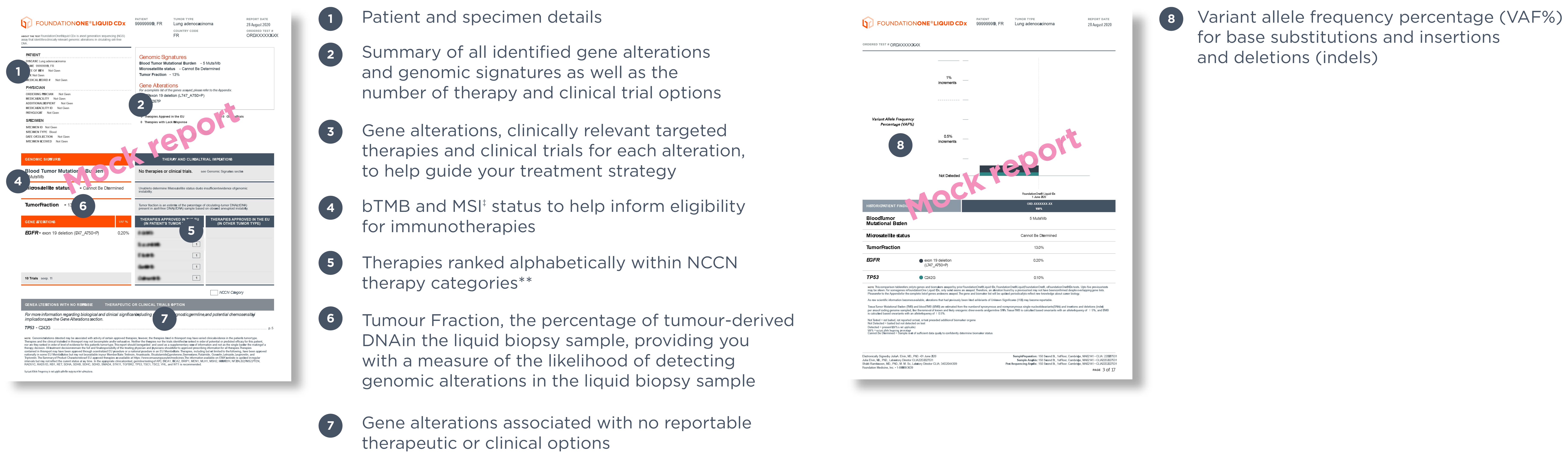
‡Foundation0ne Liquid CDx reports MSI-H status
§Validation based on overall >7,500 samples covering >30,000 unique variants across >300 genes and 37 cancer indications.1,2 To learn more about the clinical and analytical validation of FoundationOne Liquid CDx, click here.
¥75 genes are baited with enhanced sensitivity for all variant types (selected based on increased actionability with current or future targeted therapies; for more information of these 75 genes, please refer to our full gene list): other genomic regions are baited with high sensitivity.
¶Clinical validation based on evidence gathered using an earlier version of Foundation Medicine's current liquid biopsy service, FoundationOne Liquid CDx. For concordance results between these two tests, please see our full intended use at www.foundationmedicine.com/F1LCDx.
‡Therapies contained in the EU version of the report may have been approved through a centralised EU procedure or a national procedure in an EU Member State.
**For additional information on the NCCN categories please refer to the NCCN Compendium® (www.nccn.org).
bTMB, blood Tumour Mutational Burden; cfDNA, circulating cell-free DNA; CGP, comprehensive genomic profiling; ctDNA, cell-free tumour DNA; d, days; FDA, US Food and Drug Administration; MSI, Microsatellite Instability; NCCN, National Comprehensive Cancer Network; NGS, next generation sequencing; NSCLC, non-small cell lung cancer.